Video title animates on screen.
On-Screen Text: Mechanism of Action of REYVOW™ (lasmiditan) (C-V) Tablets
Voiceover: Mechanism of action of REYVOW (lasmiditan) controlled substance schedule 5 tablets
Chapter title animates on-screen.
On-Screen Text: CHAPTER 1 Migraine and Serotonin (5-HT) 1F Receptors
Voiceover: Chapter 1 – Migraine and Serotonin (5-HT) 1F Receptors
We see a woman in pain. The brain is beginning to fade in. The camera will pan around to show a side view.
Voiceover: Migraine is a debilitating neurological disease.1-4
We show the woman and her brain and nervous system anatomy. As we talk about the path of pain, the anatomy will be highlighted.
On-Screen Text: Trigeminovascular pain pathway Labels: Meninges, Trigeminal ganglion, Trigeminal nerve, Thalamus, Brain stem nuclei, eg, trigeminal nucleus caudalis
Voiceover: Migraine involves the trigeminovascular pain pathway, which consists of peripheral nerve endings that send signals from the meninges covering the brain to the trigeminal ganglion; the signal then continues on to the central nervous system brain stem nuclei.1,5,6
We see a neuron signaling above a blood vessel, which is pulsing to indicate inflammation.
On-Screen Text: Neurogenic inflammation Labels: Neuron, 5-HT1F receptor, Blood vessel
Voiceover: Nerve activity leading to the release of neuropeptides and neurotransmitters is thought to exacerbate neurogenic inflammation and nociceptor pain signaling in migraine.1,5,7
In the neuronal environment we see synapses and neuropeptide release. We see nerve endings with 5-HT1F receptors. There is a lot of signaling activity in the background.
On-Screen Text: Nociceptor pain signaling Labels: Neuron, 5-HT1F receptor, Neurotransmitters
Voiceover: Serotonin (5-HT) 1F receptors may play a role in migraine.5,8 5-HT1F receptors are involved in modulating pain signaling and are present on both peripheral and central pain pathways.7,9
We see the woman’s anatomy. The 5-HT1F receptor lights up when activated and stops the pulse from going further down the nerve. Also the background signaling is reduced when agonist binds to the 5-HT1F receptor.
On-Screen Text:
- Inhibiting neurotransmitter and neuropeptide release
- Thereby inhibiting their local activity and downstream neuronal signaling
Labels: Serotonin, 5-HT1F receptor
Voiceover: Preclinical studies have shown that activation of 5-HT1F receptors inhibits the release of neurotransmitters and neuropeptides, inhibits pain pathways, including the trigeminal nerve, and does not cause vasoconstriction of blood vessels.5,7,10
The REYVOW logo animates on-screen, followed by the rest of the on-screen text.
On-Screen Text: INDICATION: REYVOW is indicated for the acute treatment of migraine with or without aura in adults. Limitations of Use: REYVOW is not indicated for the preventive treatment of migraine. REYVOW may cause significant driving impairment. Please see Important Safety Information for REYVOW and links to Prescribing Information and Medication Guide below.
Voiceover: INDICATION: REYVOW is indicated for the acute treatment of migraine with or without aura in adults. Limitations of Use: REYVOW is not indicated for the preventive treatment of migraine.
Chapter title animates on-screen.
On-Screen Text: Chapter 2 REYVOW (lasmiditan) is a high-affinity serotonin (5-HT) 1F receptor agonist. The first and only FDA-approved ditan.
Voiceover: Chapter 2 – REYVOW (lasmiditan) is a high-affinity serotonin (5-HT) 1F receptor agonist. The first and only FDA-approved ditan.11
We see a hero shot of REYVOW flying in.
On-Screen Text: REYVOW is the first and only FDA-approved high-affinity serotonin (5-HT) 1F receptor agonist. Labels: REYVOW, 5-HT1F receptor
Voiceover: REYVOW is the first and only FDA-approved high-affinity serotonin (5-HT) 1F receptor agonist.11
We see REYVOW binding to the 5-HT1F receptor. The receptor lights up when bound.
Labels: REYVOW, Neuron, 5-HT1F receptor
Voiceover: REYVOW presumably exerts its therapeutic effects in the treatment of migraine through agonist effects at the 5-HT1F receptor; however, the precise mechanism is unknown.11
We see a blood vessel with REYVOW molecules crossing the blood-brain barrier to enter the central nervous system.
On-Screen Text: IN PRECLINICAL STUDIES:
- Although not demonstrated in human, REYVOW was shown to cross the blood-brain barrier in animal models
- REYVOW was shown to be lipophilic based on in vitro assays
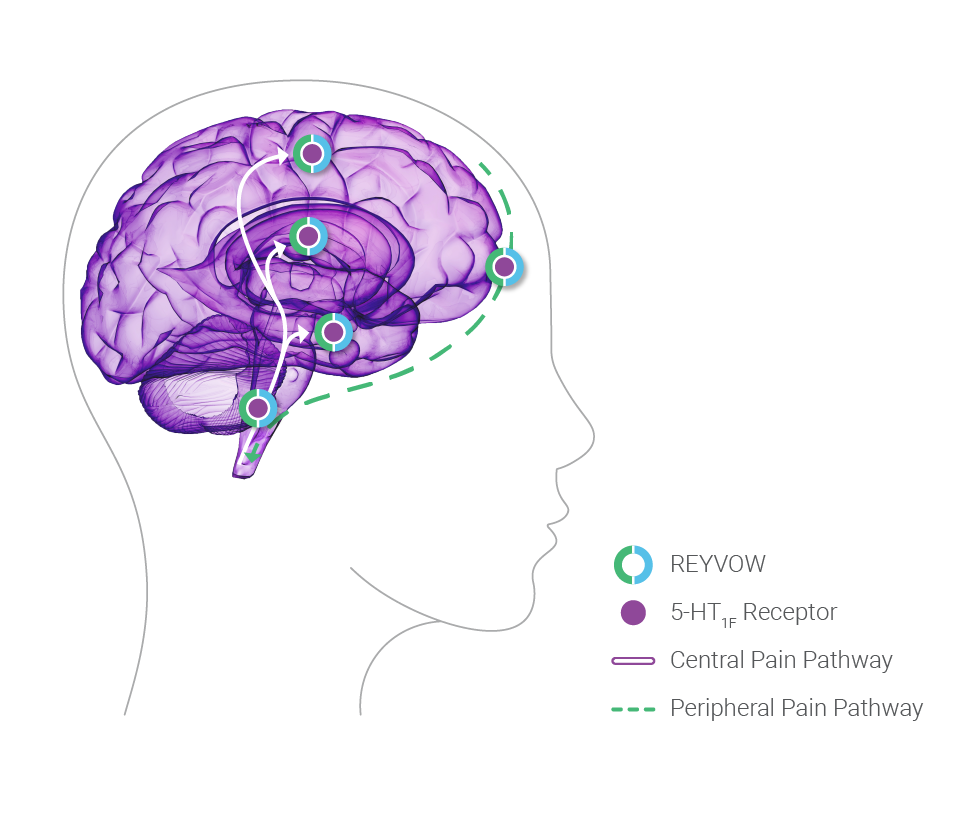
Based on the location of the 5-HT1F receptors, REYVOW is believed to act both centrally and peripherally.
Labels: REYVOW
Voiceover: Although not demonstrated in human, REYVOW was shown to cross the blood-brain barrier in animal models.12 REYVOW was shown to be lipophilic based on in vitro assays.12 Based on the location of the 5-HT1F receptors, REYVOW is believed to act both centrally and peripherally.9
The background signaling is reduced when REYVOW binds to the 5-HT1F receptor. The 5-HT1F receptor lights up when activated and stops the pulse from going further down the nerve.
Labels: REYVOW, 5-HT1F receptor, Neuron
Voiceover: REYVOW (lasmiditan) is a high-affinity serotonin (5-HT) 1F receptor agonist. It is the first and only FDA-approved ditan.11
We see the woman again.
On-Screen Text: REYVOW (lasmiditan) is a high-affinity serotonin (5-HT) 1F receptor agonist. The first and only FDA-approved ditan.
Voiceover: REYVOW is indicated for the acute treatment of migraine with or without aura in adults.11
References on-screen.
On-Screen Text:
References
- Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol. 2013;75:365-391.
- Viana M, Sances G, Ghiotto N, et al. Variability of the characteristics of a migraine attack within patients. Cephalalgia. 2016;36(9);825-830.
- Monteith TS, Goadsby PJ. Acute migraine therapy: new drugs and new approaches. Curr Treat Options Neurol. 2011;13(1):1-14.
- Sutherland HG, Griffiths LR. Genetics of migraine: insights into the molecular basis of migraine disorders. Headache. 2017;57:537-569.
- Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffman J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97:553-622.
- Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxixol. 2015;55:533-552.
- Rubio-Beltrán E, Labastida-Ramirez A, Villalón CM, MaassenVanDenBrink A. Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther. 2018;186:88-97.
- Ramadan N, Skljarevski V, Phebus L, Johnson K. 5-HT1F receptor agonists in acute migraine treatment: a hypothesis. Cephalalgia. 2003;23:776-785.
- Vila-Pueyo M. Targeted 5-HT1F therapies for migraine. Neurotherapeutics. 2018;15:291-303.
- Ahn SK, Khalmuratova R, Jeon SY, et al. Colocalization of 5-HT1F receptor and calcitonin gene-related peptide in rat vestibular nuclei. Neurosci Lett. 2009;465:151-156.
- REYVOW [Prescribing Information]. Indianapolis, IN: Lilly USA, LLC.
- Data on file. Indianapolis, IN: Lilly USA, LLC. DOF-LM-US-0019.
The REYVOW logo animates on-screen, followed by the rest of the on-screen text.
On-Screen Text: INDICATION: REYVOW is indicated for the acute treatment of migraine with or without aura in adults. Limitations of Use: REYVOW is not indicated for the preventive treatment of migraine. REYVOW may cause significant driving impairment. Please see Important Safety Information for REYVOW and links to Prescribing Information and Medication Guide below.
Voiceover: INDICATION: REYVOW is indicated for the acute treatment of migraine with or without aura in adults. Limitations of Use: REYVOW is not indicated for the preventive treatment of migraine. REYVOW may cause significant driving impairment. Please see Important Safety Information for REYVOW and links to Prescribing Information and Medication Guide below.